Conforma is being successfully used in Fiji, and with other projects in the pipeline, we are excited about the potential for one of our latest open-source software to support lower- and middle-income countries with their regulatory and workflow management.
What is Conforma?
Conforma can be described as a web-based, Integrated Regulatory Information Management System (IRIMS). Its initial development has been generously funded by the Australian Government’s Department of Foreign Affairs and Trade.
Conforma’s functionality and flexibility means that it can be used in a wide range of situations. Some examples of key medicines regulation workflows that Conforma has been designed to manage include:
Workflows include: Product Registration, Licensing, Import Control, Signals Reporting, Inspections, Pharmacist Registration
However, Conforma can be easily adapted and configured to suit an organisation’s needs, particularly for managing, monitoring and reporting on complex application workflows, creating digital forms and databases, and assigning granular permissions to different users.
How do I access the demo?
We are always happy to give a demo of Conforma to your and your team. But to make things more fun – our demo server is available for you to try yourself!
The demo allows you to create your own company, enter medicine applications, adverse drug reaction reports… AND you can also experience reviewing and approving applications as an internal user.
To showcase how versatile and flexible Conforma is, we will continue to add new features to the demo as they become available.
The demo can be accessed here and can be used on Windows, macOS, and Linux desktop systems, as well as on android tablets.
More detailed information about the demo is available in our Docs. There is also guidance on how to use the demo, including logins and tutorials for some of the functions.
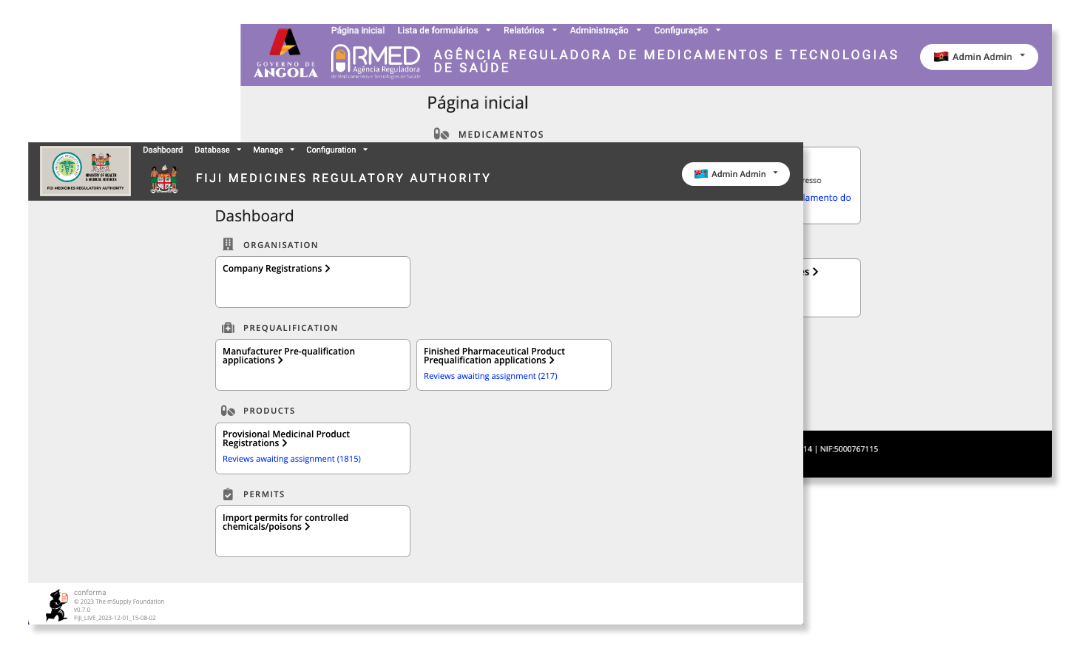
Conforma can be customised to suit the needs of different organisations such as the configurations above used by Fiji and Angola while designing Conforma to manage their medicines regulatory activities.
What if I want to know more?
We are always keen to hear from you so please don’t hesitate to reach out below – or you can email the team at support@conforma.nz.