The Fiji Medicines Regulatory Authority (Fiji MRA) has just surpassed the milestone of provisionally approving 5,000 medicines for use in-country.
This amazing feat follows two years of hard work and collaboration between the Fiji MRA and the mSupply Foundation, with Fiji utilising our new open-source regulatory information management system, Conforma.

Jess Jago from TMF (far left of photo) celebrated the launch with key local staff and representatives from the WHO and DFAT
Back in March 2023, officials from the Fijian Ministry of Health, the World Health Organization, and Australia’s Department of Foreign Affairs and Trade came together to celebrate the official launch of the Fiji MRA’s new Provisional Medicines Register.
The Provisional Medicines Register incorporates an easily accessible Online Services Portal, which runs on our open sourced regulatory management system software, Conforma.
The Online Services Portal creates a simple, digital way for sponsor companies to provisionally register their pharmaceutical products in Fiji, which in turn allows the Fiji MRA to collect important information about medicines being imported and used.

Importer companies in Fiji attending a session explaining the process of registering and submitting applications through the Online Services Portal.
TMF team member Jessica Jago says it was great to see how excited the Fiji MRA team were about the launch of the Portal, but noted that more importantly, a year down the track the team have continued to make the most of it – with 5,000 medicines provisionally approved in that time by the small Pacific nation.
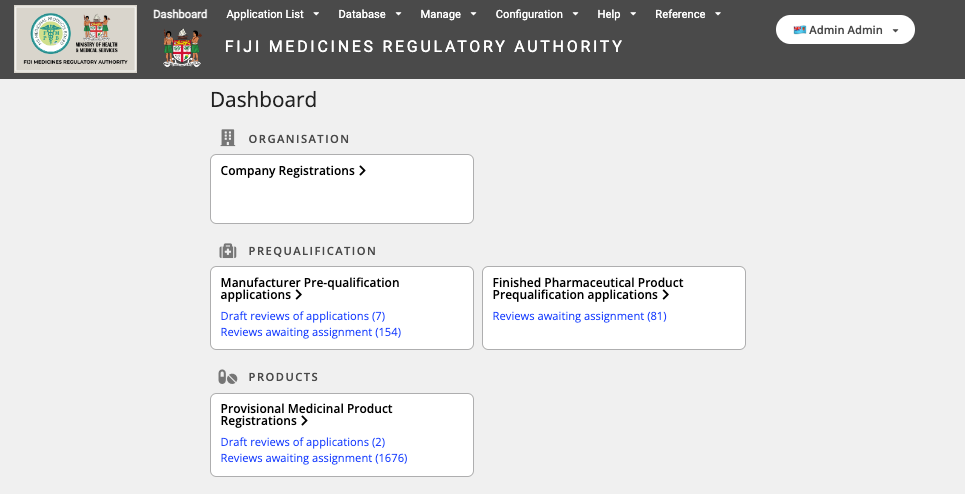
Conforma makes it easy for sponsor companies to register their products using a fully digital system, but also makes workflow management quick and efficient for regulatory authorities.
Conforma is also being used in Fiji to electronically receive applications from suppliers for controlled chemicals and poisons import permits, and more recently for manufacturer and finished product pre-qualifications to support Fiji’s medicinal product procurement processes.
The Fiji MRA team is a flagship deployment example for Conforma, now leading the way in the Pacific for many aspects of medicines regulatory processes. We look forward to Conforma’s use expanding in the Pacific and globally over the coming years. We are certain that Conforma can be an effective tool for improving medicines regulation in developing countries, ensuring access to safe and effective medicines, and improving overall health outcomes.
